Taking a new product from concept to customers in a year is difficult, and in medical devices, it’s unheard of.
But EnginuityMED of Halifax is now selling the FIVA product it dreamed up in June 2013.
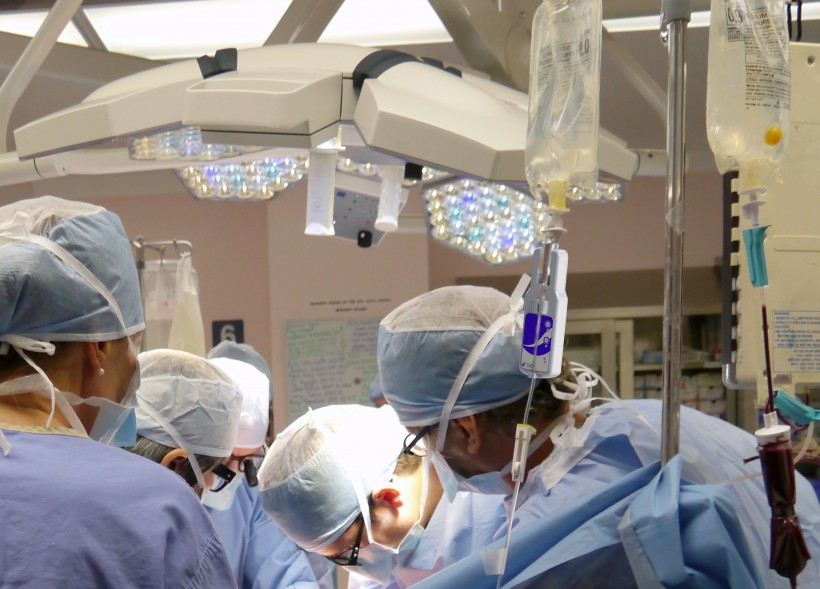
FIVA is designed to solve a very simple — and sort of obvious — problem for hospitals, especially for anesthesiologists and nurses in operating and emergency rooms. There has never been a device to tell them when a gravity-fed IV bag is empty and needs to be filled — until EnginuityMED invented one.
FIVA, which stands for Fluid Intravenous Alert, clips onto the tube at the bottom of a gravity-fed IV bag and beeps when the bag is empty. It requires no altering of the system of bags and tubes, and runs on a long-term battery. It’s now being tested at the Queen Elizabeth II Health Sciences Centre in Halifax and at other hospitals across Canada.
“The device itself is fascinating, but what is really, really exciting about this is that we went from concept to market in eight or nine months,” said Ben Garvey, a principal of the company.
The story began last summer when Orlando Hung, professor of anesthesia at Dalhousie University, pointed out the need for such a device to Barbara Campbell, the head of Hammock Facilitation, a consulting firm that advises companies on the development of medical devices.
Together, they took the project to Garvey and his colleagues at Enginuity, an engineering firm in Halifax. It had experience in design and manufacturing, but had never built its own medical device.
They ended up forming a five-member team, comprising Hung, Campbell, Garvey and two Enginuity execs, business development expert Alastair Trower and engineer Lee Babin. Thus EnginuityMED was born.
What followed was a fascinating story of resourcefulness, strategy, discipline and teamwork. It’s a textbook case of conceiving a minimum viable product, and rigorously developing it to bring in early revenue before proceeding to more sophisticated products.
With little more than a concept, Hung received $50,000 from Innovacorp’s Early Stage Commercialization Fund, which allowed early prototyping and meetings with focus groups. With their business model honed a bit, they entered I-3, Innovacorp’s competition for Nova Scotia startups. They finished in the top five for the Halifax region, and set a goal for themselves.
In preparing their January 2014 pitch for the I-3 competition, EngiunityMED decided to focus on a single, simple product that it could produce by the Canadian Anesthesiologists’ Society’s annual conference in St. John’s, N.L., in June. If they didn’t have the product ready for that conference, they’d have to wait a year to go to the next national conference.
All five members had other full-time jobs, but they managed to develop the product over the winter. That meant Babin and Enginuity engineer Gleb Sekretta produced six different prototypes. They were even able to obtain the necessary medical device establishment licensing for EnginuityMED and put the FIVA product through the regulatory approval process for a Class 1 (i.e., simplest) product with Health Canada.
The founders financed it themselves. They were working on it up till the night before Babin and Trower boarded the plane for St. John’s.
But they made the deadline and landed sales.
Now, with experience in producing and getting approval for a medical device, EnginuityMed is planning its next products. It is securing a round of financing, and is hoping to hear from other clinicians with ideas for products.
Said Campbell, “We want to see people’s concepts, vet them and see if they fit our stream of clinician-driven innovation.”