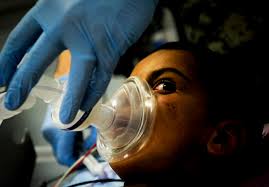
A Halifax medical technology company has a basic aim: to make anesthesia safer.
DMF Medical plans to release its signature product, Memsorb, in the next year and hopes it will improve the process of removing carbon dioxide from the system that puts patients to sleep during operations. Memsorb is a membrane-based device that replaces the collection of chemicals that is now used to remove CO2 from the process. The new device, which will go through regulatory processes in Canada and/or the European Union this winter, is safer, better for the environment and can save hospitals money.
“Anesthesia is an old, traditional business and things can be improved by eliminating problems,” co-founder and director of R&D Florentin Wilfart said at the recent Atlantic Venture Forum.
In this traditional medical process, a patient inhales a vaporized anesthetic mixed with oxygen, and exhales a combination of oxygen, anesthetic, carbon dioxide and toxins. It passes through a chemical filter to strip out the toxins and CO2, and then feeds it back into the stream of gases being delivered to the patient. Because anesthetics are so expensive, the filtered exhalation is used again to get maxiumum use out of the anesthetic.
But there are problems with this “anesthetic loop” because of the chemical reaction used to remove CO2 — mainly, it produces compounds that can be harmful to the patient.
“People don’t like to admit this is a real problem even through it’s been published in several places,” said Wilfart.
What’s more, the process spews CO2 and chemicals into the air, so the impact worldwide on the atmosphere is equivalent to the production of 4.4 million tons of CO2 a year. And the residue chemicals produce 115,000 cubic metres of solid waste a year that is expensive to dispose of. In most operating rooms, the chemical canisters must be replaced daily.
PhotoDynamic Lands Funding from FAN
DMF Medical has devised Memsorb to solve these problems. The device uses a membrane to filter out the CO2 and toxins, creating no chemical reaction. The product, which fits seamlessly into existing anesthesia equipment, lasts for several months, and is recyclable. The only thing it needs to work is a stream of oxygen, which would be readily available in any operating room.
Wilfart and a team of medical and business professionals have been working on the project for more than five years. (In 2011, DMF actually competed in the first BioInnovation Challenge, the region’s main life sciences pitching competition.) The company has raised a total of $3.1 million in capital, including private investment and government loans and grants. This included a $1.25 million loan from the Atlantic Innovation Fund in 2012.
DMF Medical now has a working prototype of Memsorb and has done clinical trials on 20 patients. It will soon be in the midst of the regulatory process to have the product approved in Canada and the EU. The first jurisdiction to grant approval will be the first market it will enter.
Assuming the manufacturer can produce Memsorb in the quantities the market demands, the group hopes to have the product on the market in 2017.